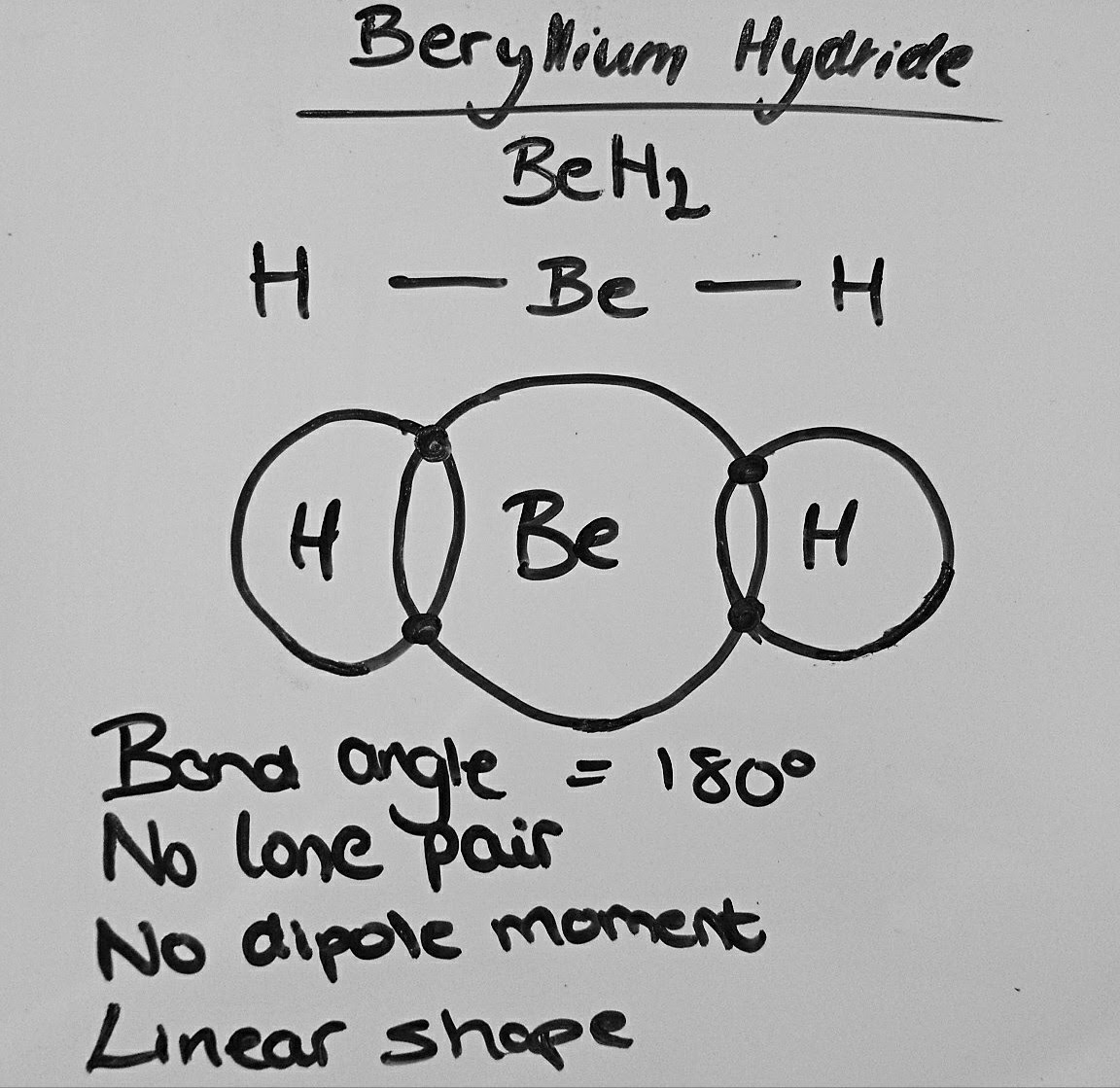
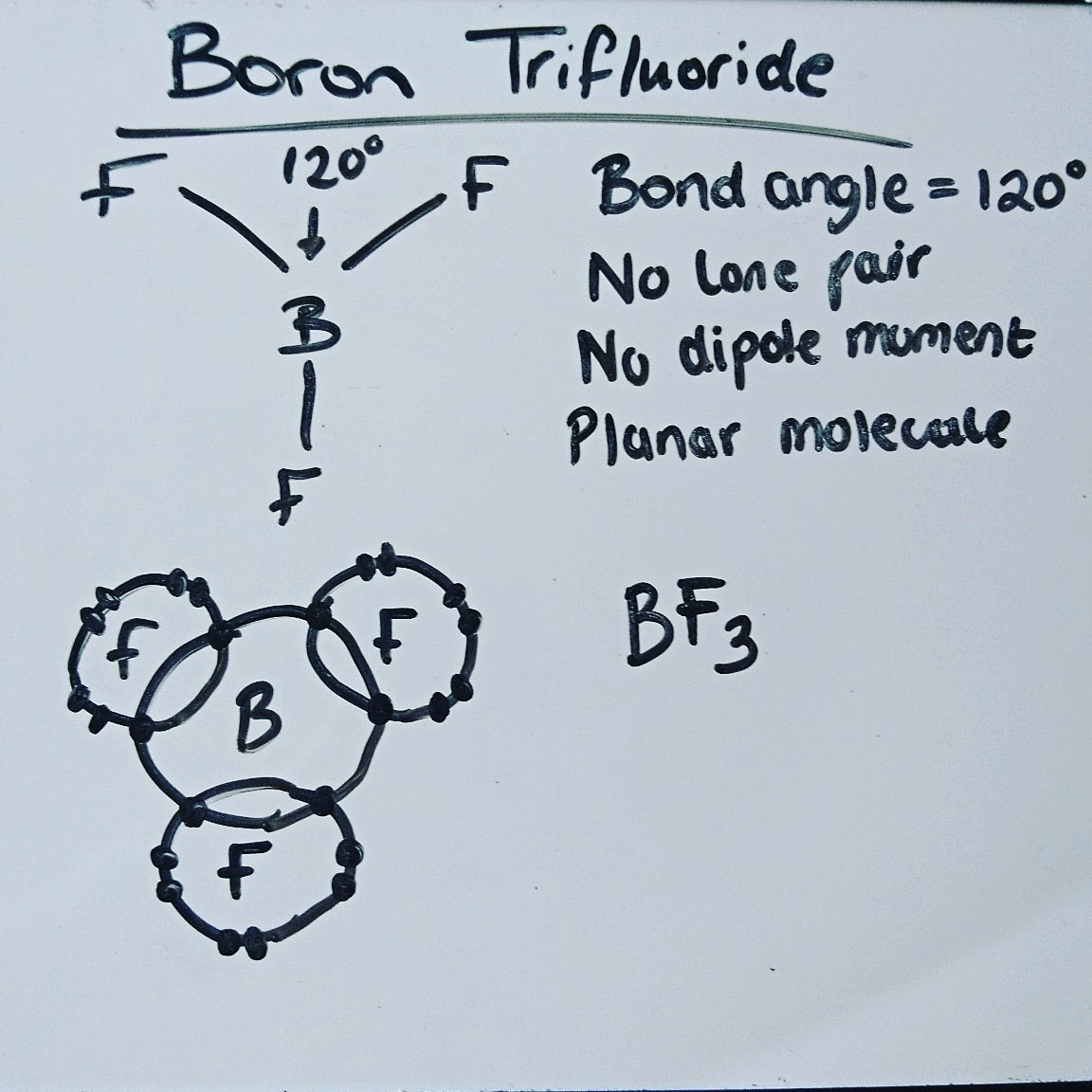
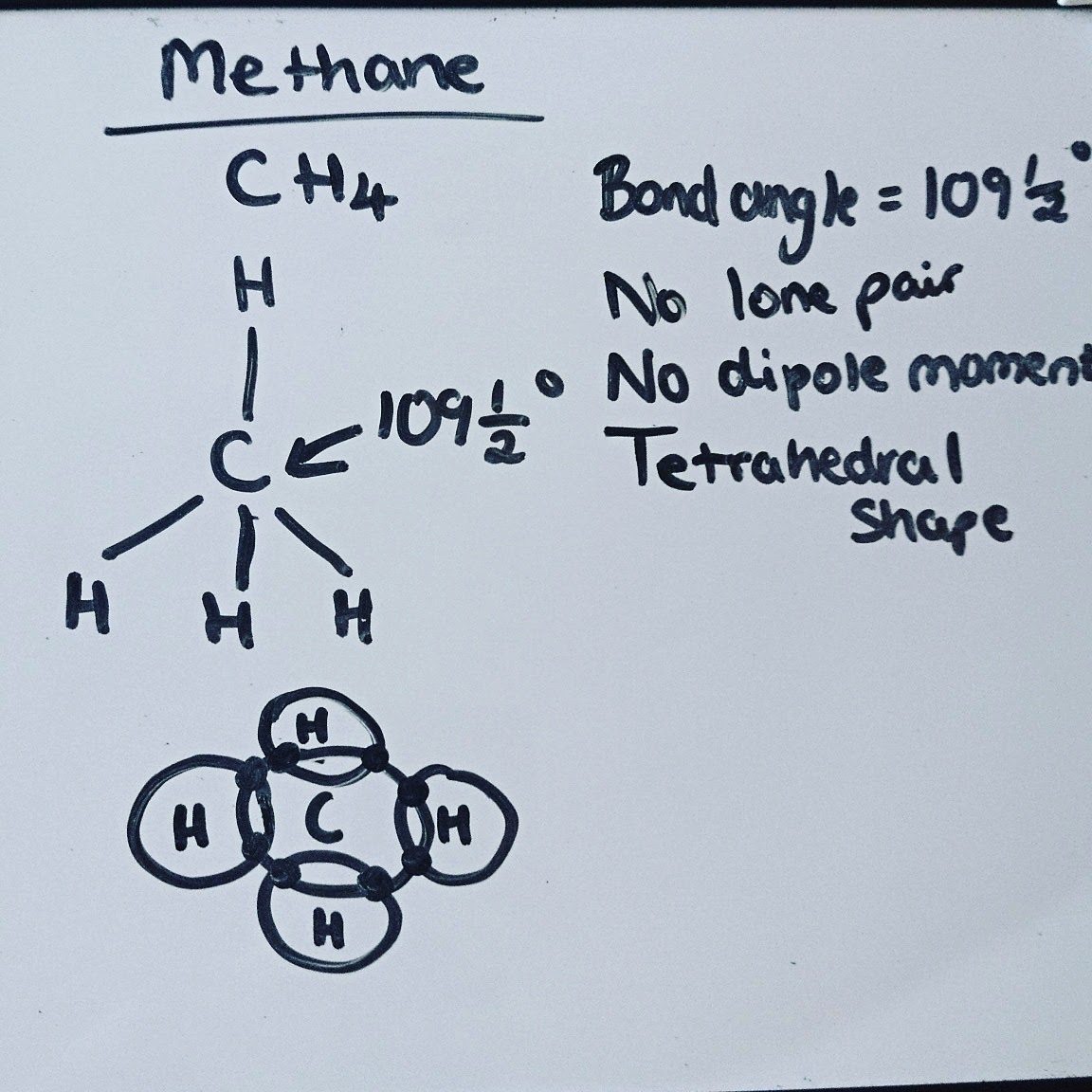
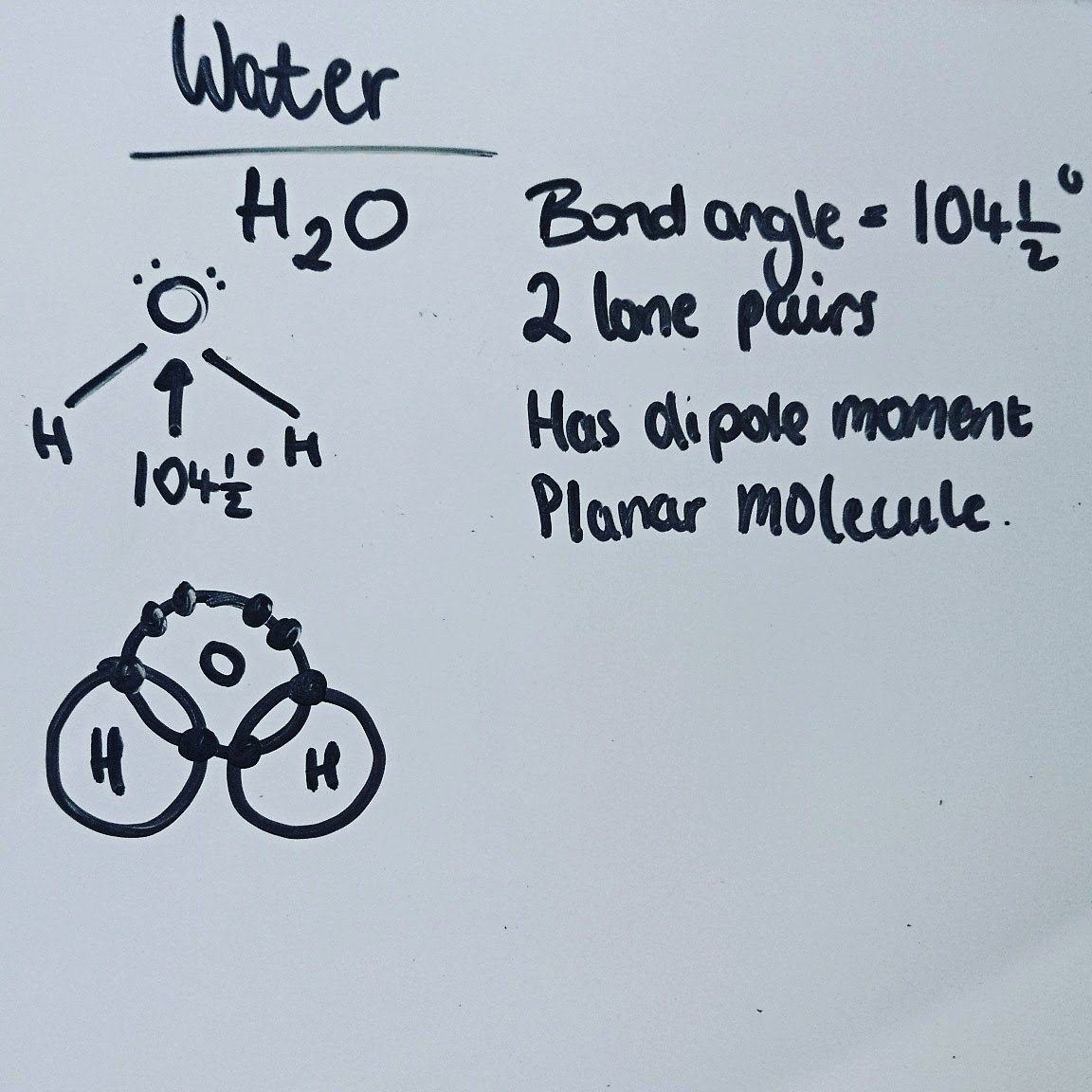
PhysChem
Chemistry
Bonding and Molecular Theory
Ion:
Bonding and Molecular Theory
Ion:An atom or a group of atoms which has a nett electric charge
Ionic Bond:The bond formed between 2 atoms when one atom takes an electron(s) completely, from the other resulting in a negative and positive ion which attreact one another
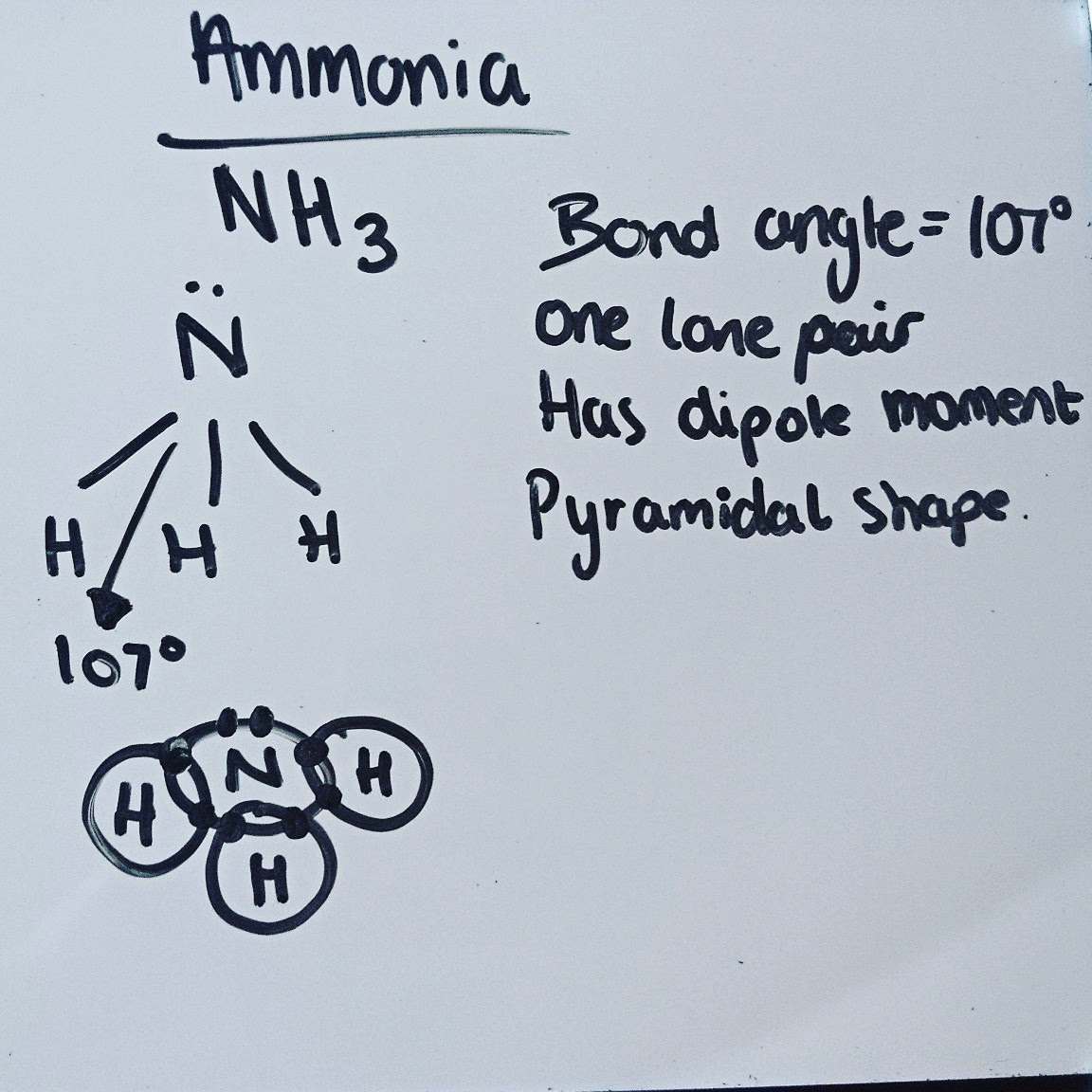
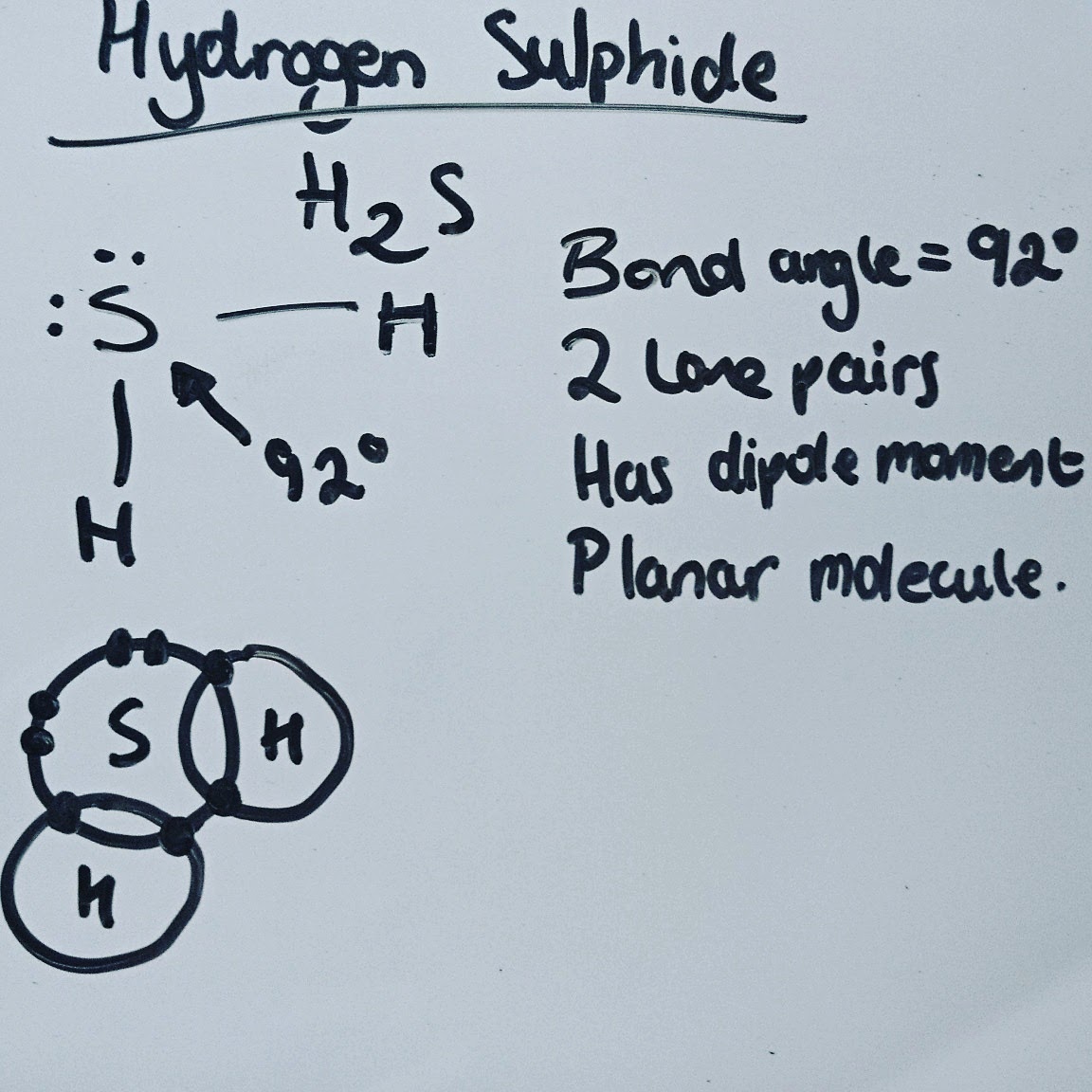
Covalent Bond:The bond formed when 2 atoms share a pair of electrons
Dative Bond:like a covalent bond but the shared pair of electrons were both contributed by the same atom
Electronegativity:is a measure of attraction of an atom of that element for a shared pair of electrons
Hydrogen Bond:when the hydrogen atom is bonded to a high electronegativity element. The hydrogen becomes slightly positive then the hydrogen bond is the bond between that hydrogen and slightly negative atom of the nett molecule
Dipole moment:forces are forces of attraction between the negative pole of one molecule and the positive pole of another
Van der Waals:forces are weak attractive forces between molecules resulting from formation of temporary dipoles
Metallic Bond:bond between the cloud of valency electrons and the positive metal ions in the metal
Crystal:a regular, solid, repeating structure of atoms, ions or molecules arranged in a lattice shape